- Home
- News and Resources
- Resources
- Submission Ready Programs: Path to Stress free Submission
Submission Ready Programs: Path to Stress free Submission
We focus on Datasets, Outputs and Study Reports, but did we ever think of the programs that used to generate these datasets and outputs. Are these programs developed in line with regulatory standards and fully compliant right from the start?
At Amarex we have made this our top priority. By developing submission ready programs from the beginning of every study, we elevate the standards of our entire submission package. This not only ensures Regulatory readiness but also delivers the highest levels of reliability and quality in every output we generate.
Why does this matter:
Quality and reliability of the data
- This ensures all required components to a program are included, and coding standards are specifically followed, resulting in generation of quality and a reliable output.
- Also enables early detection of potential issues or errors in code and outputs, which helps avoid delays in timelines while enhancing the overall quality of the output.
Timely submission of the package
- As the study programs are already developed with compliance in mind, there is no need for further improvement or updates to convert them to submission ready during filing. Thus, saving overall time
Traceability
- This is an important feature. These programs provide clear trail form source data to results, which is crucial for regulatory review and to expediate review process.
Adhoc requests and Post Submission Queries
- Developing Submission ready Programs and keeping Submission standards, makes it easy to address any post submission queries or to create adhoc requests.
Audit Readiness:
- As the programs include all required information per the Regulatory requirements, it minimizes the risk of compliance failures and ensures proper data handling.
Reproducibility:
- Maintaining programs related to various milestones in the right folders aids in reproducibility of the results i.e., Programs rerun based on the same source produce same outputs.
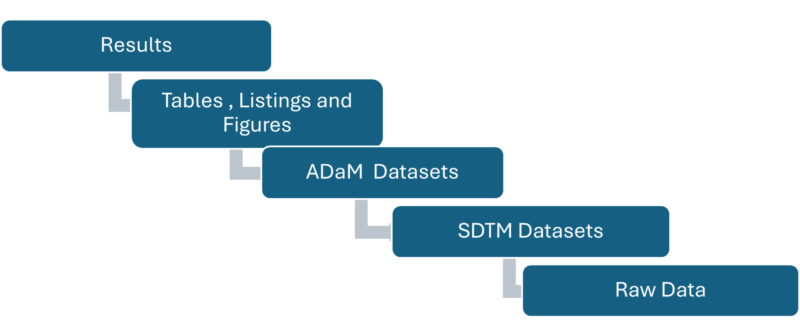
Graphical Representation showing the Advantages of Developing Submission Ready Programs right from the start:
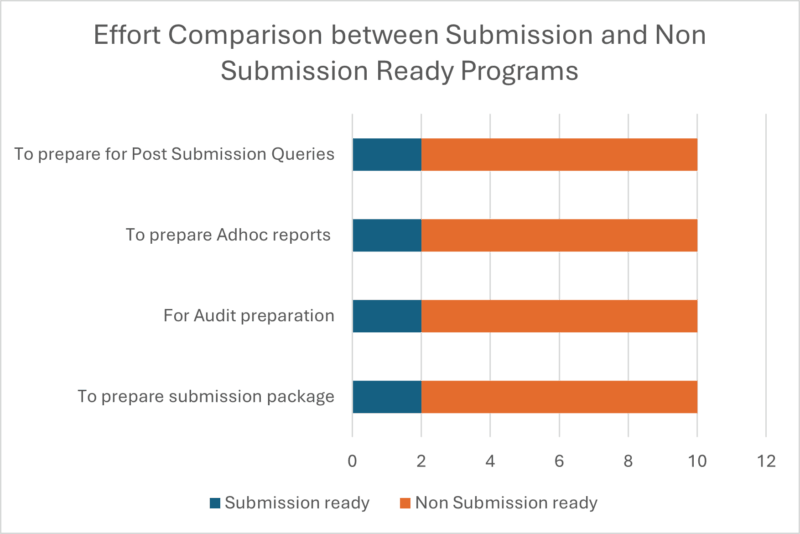
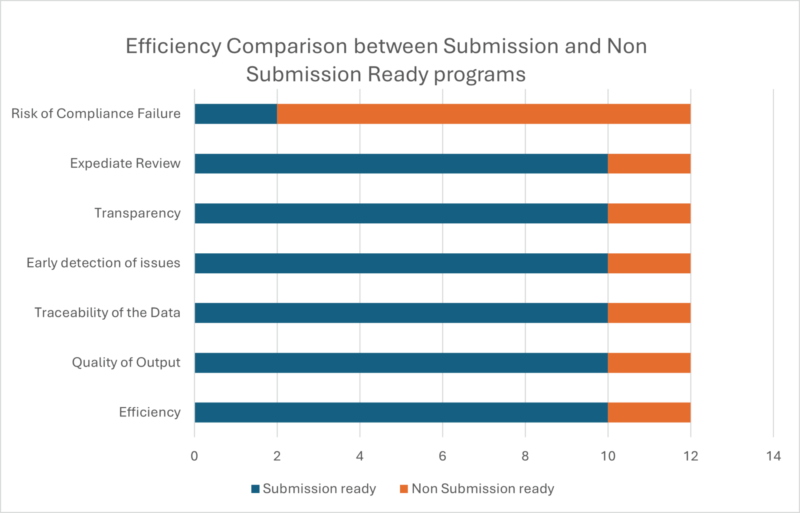
At Amarex, investing time upfront to develop submission ready programs from the very start of a study has proven to be a game changer. It not only drops the stress of last-minute package development but also ensures we are always prepared for unexpected sponsor or board requests. This approach allows for thorough logic reviews, early error detection, and consistent output generation, resulting in a streamlined, efficient, and high-quality submission package. The benefits are clear: considerable time and cost savings, smoother sponsor interactions, and an accelerated review process.
References:
- US Food & Drug Administration, Study data technical conformance guide.
- Maria Dalton, “Submission of Software Programs to Regulatory Agencies”
